LynxDx
Quick Snapshot
- Role: Head of Product overseeing laboratory information systems
- Team: Led an eight-person software engineering and systems analyst group while partnering with lab operations, compliance, and commercial teams
- Customers: University of Michigan, Trinity Health, Detroit Public Schools, skilled nursing facilities, and public health partners statewide
Mandate / Opportunity / Problem Scope
LynxDx pivoted from commercializing a novel prostate cancer diagnostic to scaling COVID PCR testing at the height of the pandemic. The challenge was to evolve a custom laboratory information management system (LIMS) that could orchestrate high-throughput testing, serve diverse enterprise clients, and meet strict HIPAA, CAP/CLIA, and NIST requirements.
What I Led / Delivered / Highlights
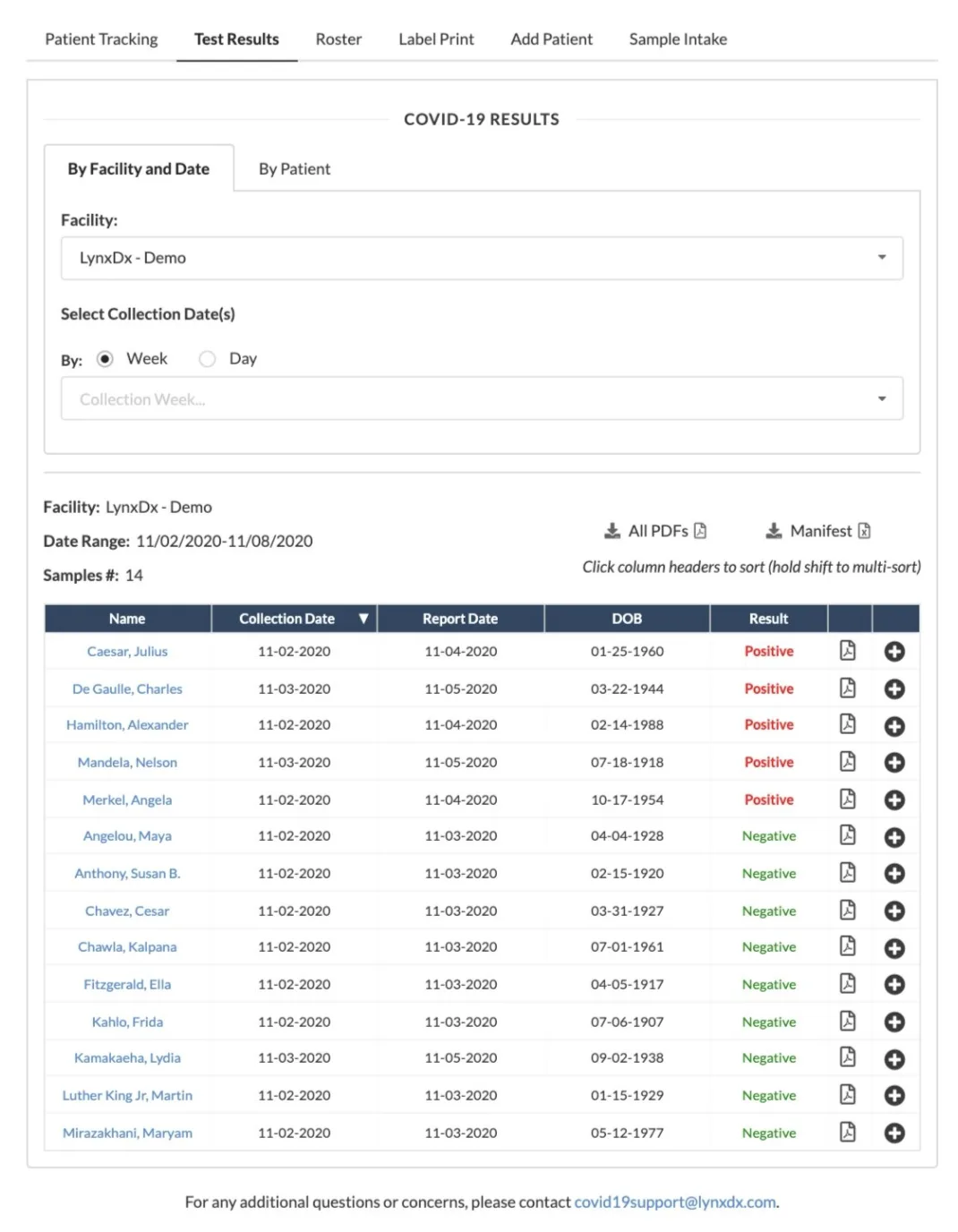
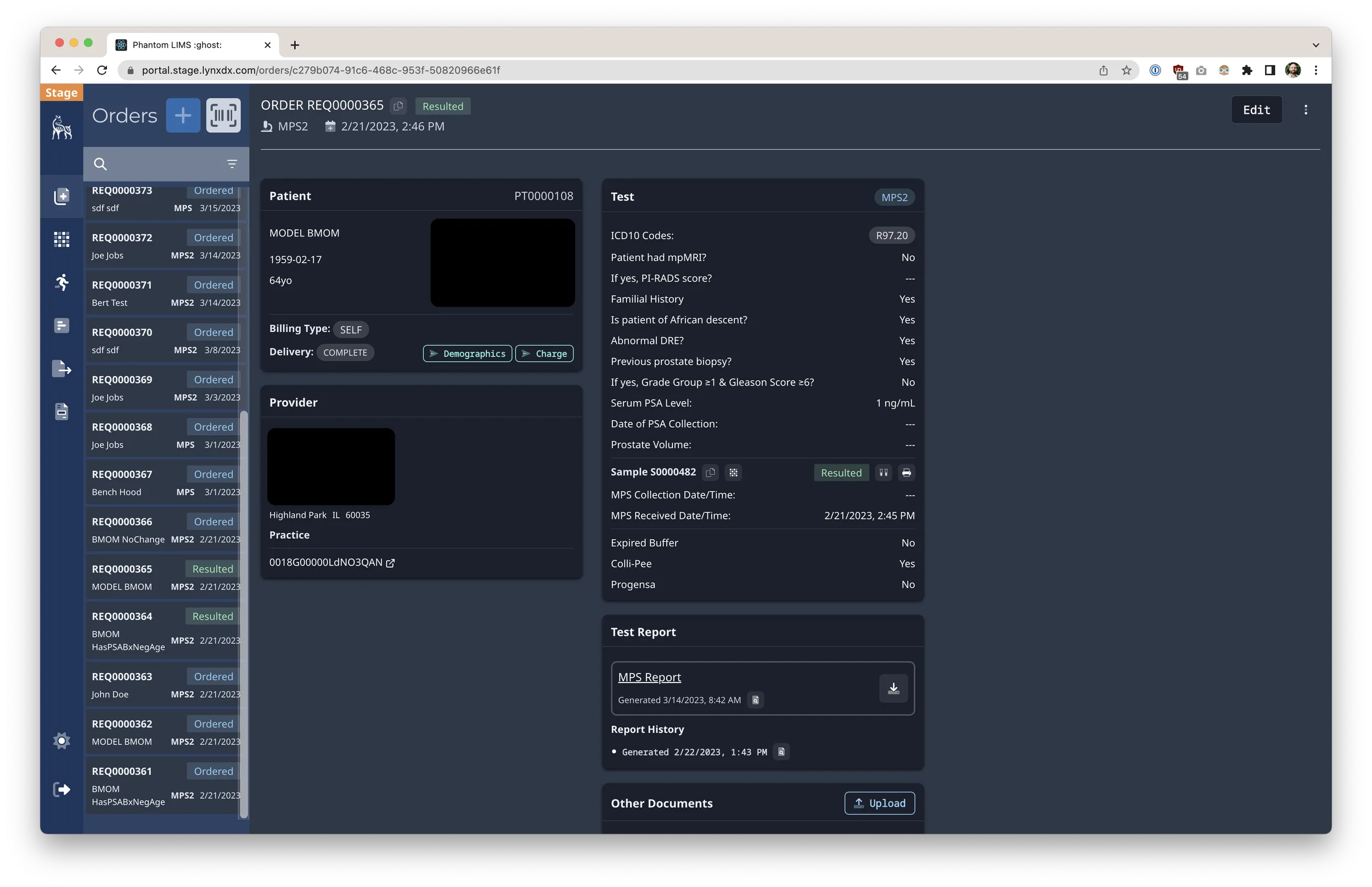
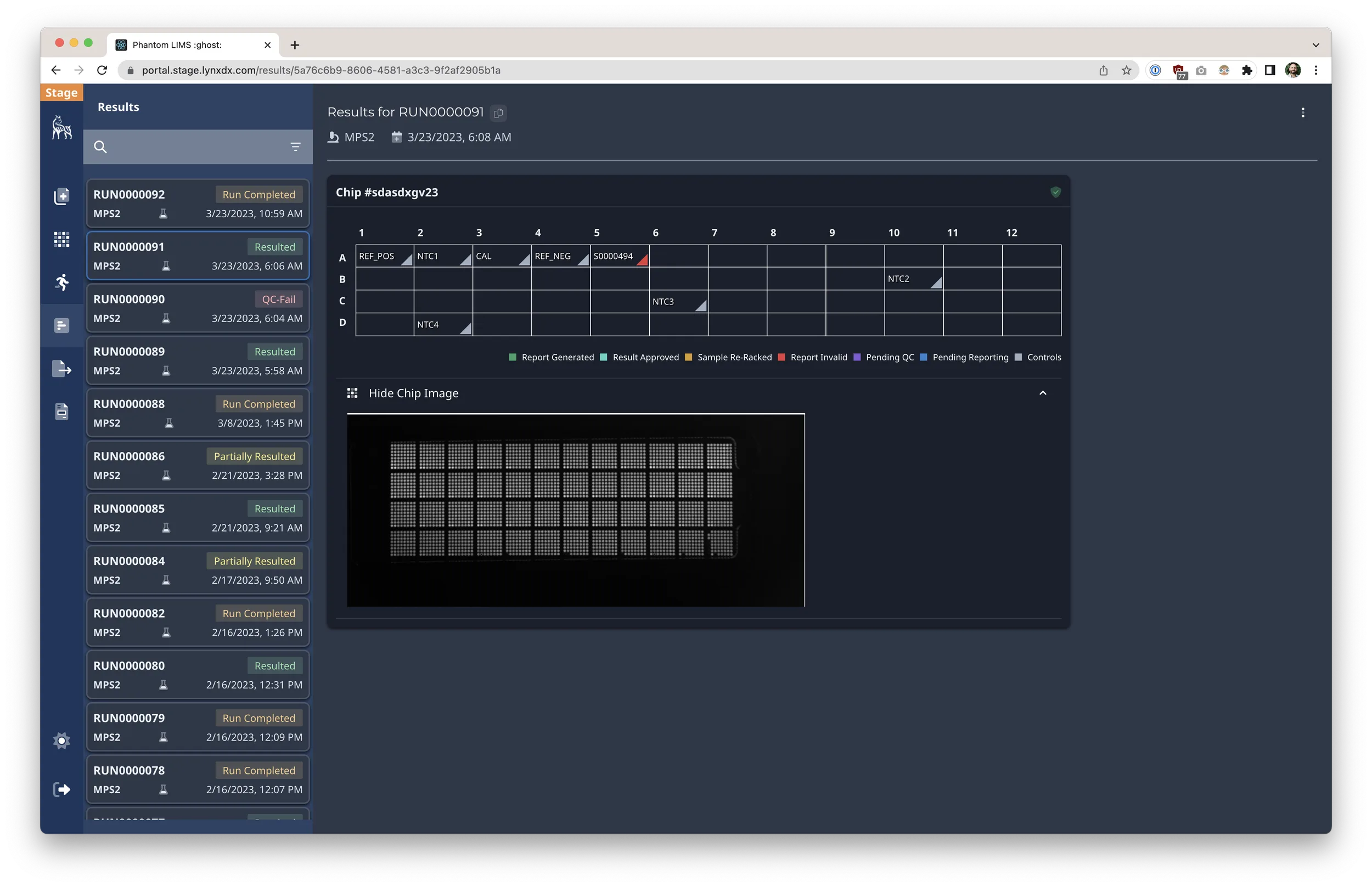
- Defined the product roadmap that took the LIMS from zero to supporting 2.5M PCR tests, with tailored workflows for nursing facilities, community drive-thrus, and university programs.
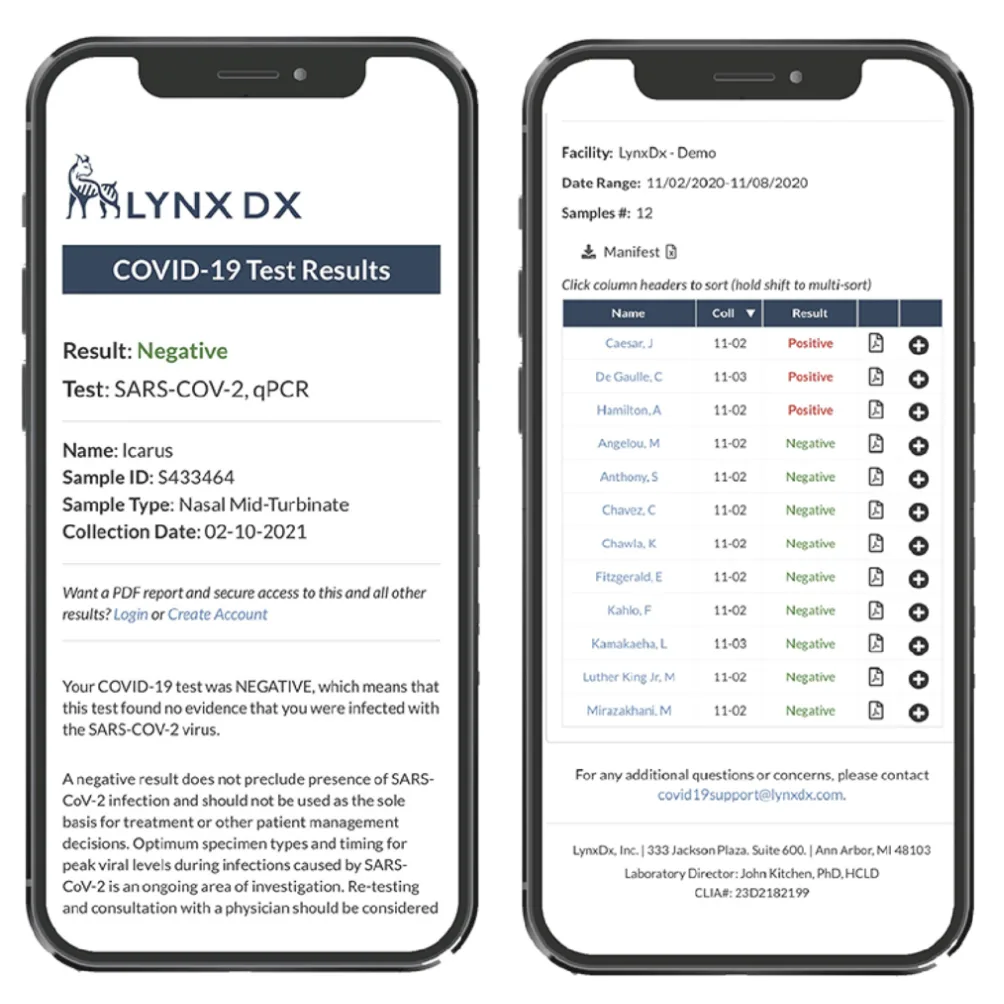
- Drove execution of net-new product capabilities including client portals, shipment tracking, metrics dashboards, and electronic health record integrations.
- Orchestrated partnerships with external IT and data teams to synchronize results delivery via secure integrations and automated reporting.
- Authored product documentation and controls that satisfied regulatory, legal, and privacy compliance expectations.
Impact / Lasting Value / Takeaway
The platform underpinned LynxDx’s rise to Michigan’s leading COVID testing lab, enabling $100M in revenue while keeping patients, administrators, and clinicians aligned on timely results. Establishing a resilient, compliant, and insight-rich LIMS created a foundation the company could extend back to its core precision diagnostics mission.